
5 key challenges when validating lyophilisers
Lyophilisation, also known as freeze drying, is a critical process step in the stabilisation of many active pharmaceutical ingredients in which aqueous solutions are transformed into powder via sublimation. The lyophilisation cycle, consisting of initial freezing, primary drying, and secondary drying can present many challenges which can be identified via thorough validation. In this article, we aim to cover the most common challenges and how you would go about identifying them.
1. Cross-Contamination Concerns:
Ensuring aseptic conditions and preventing cross-contamination is a major challenge during lyophilisation. The lyophilisation equipment and its processes should be designed to ensure that product or material sterility is maintained during lyophilisation by preventing microbial and particle contamination between the filling of products for lyophilisation, and completion of lyophilisation process. The risk of contamination arises during loading and unloading of vials, especially in multi-product facilities. Contamination risk can be identified and/or validated using swabbing/ surface testing, dye tracing, leak rate testing, airborne particle monitoring and validation of cleaning procedures. It is essential that these risks are identified and quantified as they can directly affect patient safety.
2 . Inconsistent Cycle Development
Inconsistent cycle development during lyophiliser validation can lead to variations in product quality and stability. There are many variables during the freezing phase, primary and secondary drying phases which can affect the final product. For example, variations in temperature can compromise the uniformity of product drying, potentially resulting in non-homogeneous products with varying degrees of moisture content. Detecting changes in these variables and their effect on the final product is crucial to ensuring that the lyophilisation process is reliable and reproducible. Cycles can be developed by temperature and pressure mapping and in process sampling for moisture.
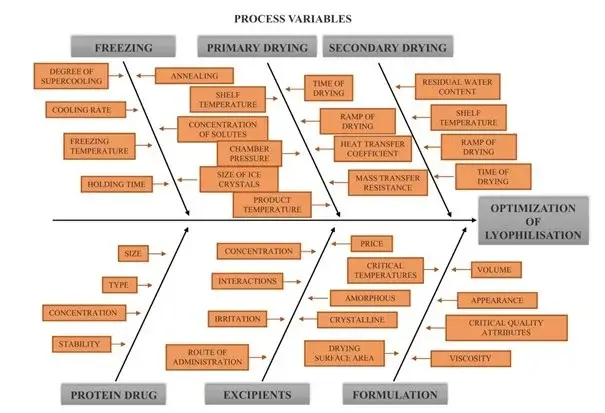
Formulation and process variables affecting critical quality attributes of lyophilised products
3. Vacuum Problems:
Ensuring an integral chamber and maintaining accurate vacuum levels is crucial for achieving effective drying during the secondary drying phase. Inaccurate vacuum levels can prolong the drying process, effect the removal of bound water, and ultimately impact product stability. If the vacuum pressure is insufficient, it can lead to compromised moisture removal potentially resulting in residual moisture in the final product. This in turn causes reduced shelf life and compromised product quality. Evacuation rates and lowest achievable pressures are standard tests that indicate the performance of the vacuum-pumping system.
4. Temperature problems
It is important to validate the temperature changes in the heat transfer system and shelving over time in order to ensure batch uniformity. For example, the empty chamber must be mapped to ensure the shelf surface temperature at any spot on one shelf or across shelves is within±0.5 °C of the average after equilibration (though this value may differ depending on the requirements of the product). Measuring the cooling rate over time is also important as it can affect ice crystal formation and subsequent drying, with slower cooling rates being associated with larger ice crystal formation.5. Sensor Inaccuracies:
Temperature and pressure sensors play a vital role in monitoring and controlling the lyophilisation process. Inaccurate sensors can provide misleading data, leading to incorrect adjustments of process parameters. This can result in compromisation of the product which can negatively impact product quality, potency, and stability. These problems can be mitigated by regular calibration and validation to required standards.
Conclusion
Discrepancies in equipment performance can have far-reaching consequences on the final product quality, for example variations in temperature, vacuum, or sensor readings can lead to non-uniform drying across vials or containers within a batch. This non-uniformity can result in a mix of under dried and overdried product, leading to inconsistent potency and efficacy, which ultimately can affect patient safety. The risk of microbial contamination and subsequent potential to affect patient safety also cannot be underestimated To mitigate these challenges, validation managers should implement a comprehensive lyophiliser validation plan in addition to regular equipment maintenance, calibration, and real-time monitoring. Additionally, close collaboration between process development, engineering, and quality assurance teams is crucial to address equipment discrepancies effectively. By proactively addressing equipment performance issues, validation managers can ensure the reliability, reproducibility, and overall quality of the lyophilisation process and its final products.
Our team at BPV, have the skills and expertise to identify and resolve all these common problems through extensive testing, optimisation and development of Clean In Place, Steam in Place and Lyophilisation processes. To find out more about our validation services, please get in touch with us at [email protected]
1. Bjelošević, M., Pobirk, A.Z., Planinšek, O. and Grabnar, P.A., 2020. Excipients in freeze-dried biopharmaceuticals: Contributions toward formulation stability and lyophilisation cycle optimisation. International journal of pharmaceutics, 576, p.119029.
2. European Comission (2022). The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. [online] Available at: https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf [Accessed 29 Aug. 2023].
3. Trappler EH. Validation of lyophilisation. InPharmaceutical process validation 2003 Mar 27 (pp. 332-371). CRC Press.
4. Jameel F, Alexeenko A, Bhambhani A, Sacha G, Zhu T, Tchessalov S, Sharma P, Moussa E, Iyer L, Luthra S, Srinivasan J. Recommended best practices for lyophilisation validation 2021 part II: process qualification and continued process verification. AAPS PharmSciTech. 2021 Nov;22:1-25.

