BPV are UK validation experts offering thermal mapping services to UK and European life science businesses in regulated industries, such as pharmaceuticals. We’re a trusted team that can support regulatory compliance, as well as ensuring product quality across storage, transport, and processing environments remains at its best.
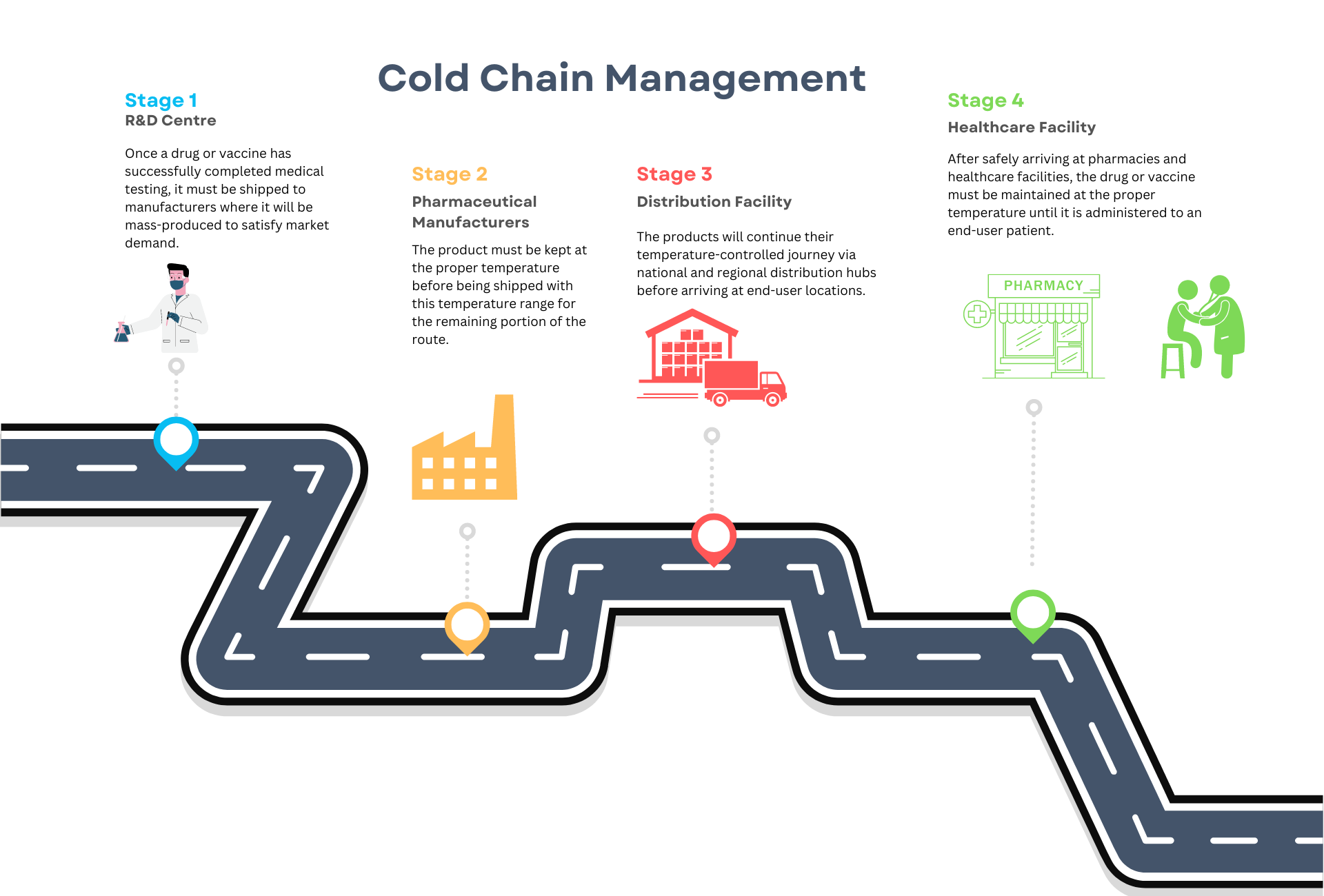
At BPV, we provide comprehensive thermal mapping services to monitor temperature and humidity in pharmaceutical warehouses and other storage facilities. We provide guideline-referenced and GxP-compliant implementation of temperature mapping as part of Good Manufacturing Practice (GMP).
Our temperature mapping services ensure that fluctuations in temperature and humidity are prevented, safeguarding product quality in your facilities. Temperature and humidity can significantly impact both raw materials on the manufacturing floor and finished goods in your warehouse, making it essential to have reliable monitoring and control measures in place.
Our experienced team utilises proven and reliable data loggers, thermocouples, and probes to continuously map temperatures across all areas of your storage facilities. This allows our team to identify potential hot spots and cold spots to ensure conditions stay within validated ranges as per your requirements and specifications.
If you’re in need of thermal mapping services, get in touch with us today to discuss your specific needs with a friendly member of our team, or to request a quote.
What is Thermal Mapping?
Thermal mapping evaluates the temperature distribution within a controlled environment by strategically placing temperature sensors around the room to identify potential hot or cold spots. It ensures that temperature sensitive products are stored within the required temperature range, maintaining their integrity, safety, and legal compliance.