How Freeze Drying Works
Lyophilisation, or freeze drying, is an essential procedure in the pharmaceutical and biotechnology sectors, with over one third of biopharmaceuticals currently being lyophilised. It has become increasingly crucial for the preservation and stabilisation of sensitive biological materials, such as proteins, enzymes, and other active pharmaceutical ingredients (APIs), as well as for the extension of the expiration life. Long-term stability is increased in the freeze-dried solid form because chemical or physical degradation reactions are either prevented or greatly slowed down and the probability of microbial growth is reduced. While freeze drying offers numerous benefits, the process can be very energy, time and cost heavy which presents a need for process optimisation. By understanding the processes involved, those involved in dealing with biopharmaceuticals, whether in a research, production, or quality control capacity can work to optimise this essential process. This article aims to provide a comprehensive understanding of how freeze-drying works covering the fundamental principles, process steps involved and an overview of the validation process.
The Basics of Freeze Drying
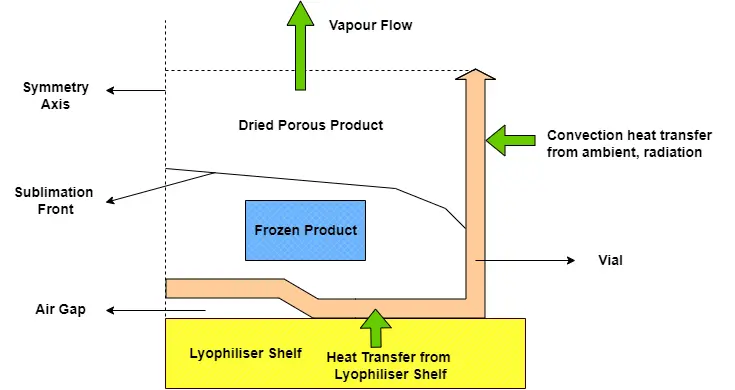
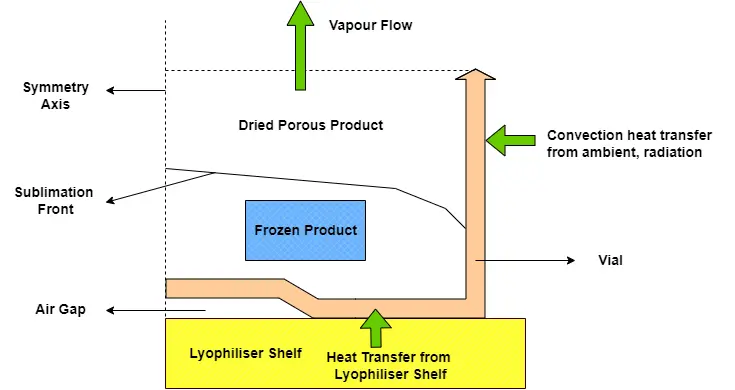
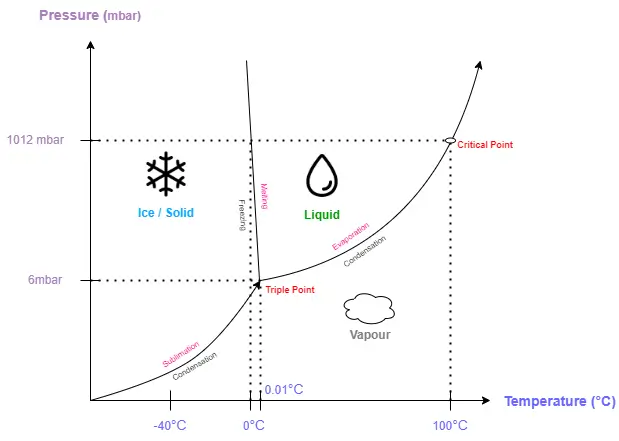
The fundamental principle of freeze drying is a dehydration technique that extracts water from a frozen product through sublimation which is the process by which a solid transitions directly into a gas, bypassing the liquid phase. This process is facilitated by precise vacuum and temperature conditions within the lyophiliser. The temperature at which sublimation occurs is reduced by the low-pressure (vacuum) environment, which facilitates the sublimation process and transitions water from the solid phase to the vapour phase. The structure and bioactivity of sensitive materials are preserved by this gentle approach to water removal, rendering it invaluable for the handling of delicate substances. Freeze drying is broken down into three stages: freezing, primary drying, and secondary drying which are discussed below. Each stage is crucial to the finished product’s quality and plays a significant role in the overall process.
The Three Stages of Freeze Drying
Freezing Stage
Initially, lyophilisation involves the chilling of a product at a temperature of approximately -40°C under atmospheric pressure. During this phase, liquid nitrogen is frequently chosen as a rapidly deployed, environmentally friendly, and cost-effective refrigerant. The aqueous solution, which contains the API and a variety of excipients, is chilled during this freezing phase until an amorphous crystalline or combined amorphous-crystalline solid matrix is formed. In general, this involves the filling of the solution into glass containers and placement onto temperature-controlled shelves. The use of temperature-controlled shelves enables the precise regulation of cooling rates, which consequently effects the freezing rates of the product and the size of the crystals.
Primary Drying Stage
The primary drying stage is frequently regarded as the most critical phase of the freeze-drying process. In process development, the primary drying stage is commonly targeted for its potential to significantly reduce the overall cycle time. Moreover, if the primary drying stage is completed under suboptimal circumstances, it may result in substantial product defects. During this stage a vacuum is pulled at reduced temperatures, to facilitate the sublimation of ice into water vapour bypassing the liquid phase. As the frozen product’s ice sublimates, a porous structure results which is often referred to as the desiccated layer. In order for the sublimation process to take place, two fundamental conditions must be satisfied:
- Sublime steam must be continuously removed from the sublimation area, maintaining the differential pressure of the vapour, which consequently removes water steam from the chamber.
- Heat required must be continuously supplied to the product through the lyophilisers shelves to balance out the energy loss from product during sublimation. The quantity of heat energy that is supplied during the sublimation process should be equivalent to the amount of energy required for the ice to sublimate.
A variety of factors, such as the temperature difference between the product and the condenser, the chamber pressure, and the resistance of the desiccated layer to vacuum flow influences the rate of sublimation during primary drying. Appropriate management of these parameters guarantees that the quality of the product is not compromised during the drying process.
Secondary Drying
The final stage of freeze drying is secondary drying, which involves the removal of unfrozen attached water through moisture desorption. This stage is conducted under high vacuum conditions and slightly higher temperatures. This procedure eliminates any residual moisture that remains attached to the product following the sublimation of the ice. The objective is to decrease the moisture content to a level that guarantees the product’s long-term stability. The shelf temperature is frequently elevated to a temperature range of 20°C to 40°C for a period of several hours to facilitate diffusion, as shown in the graphic below. The drying rate is based upon the thickness of the material and the size of the freeze-dried material. 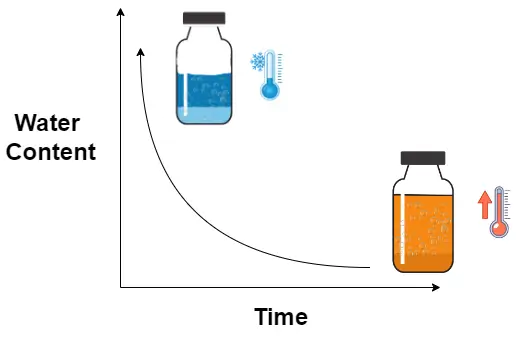 Upon completion of these three processes, the product typically should contain no more than 1% water and has a porous shape. Near the end of this stage, a sampling procedure can be executed utilising a sample extractor (or sample thief) to extricate a small number of vials from the freeze dryer during the drying process. These samples are subsequently analysed for moisture content. After the target water content is achieved, the drying phase is then concluded. The analysis of lyophiliser samples for moisture content can be a part of standard operating procedures at some laboratories; however, the process needs to be done carefully; otherwise, it can potentially compromise the sterility of the batch. Another issue with this procedure is that vials sampled at the front door are commonly observed to dry at a significantly faster rate than the vials in the centre of the tray, a phenomenon known as “atypical drying”. It is important to note that, as of today, there is no available technology or sampling of vials in large-scale freeze drying to determine the product’s secondary drying progress over time.
Upon completion of these three processes, the product typically should contain no more than 1% water and has a porous shape. Near the end of this stage, a sampling procedure can be executed utilising a sample extractor (or sample thief) to extricate a small number of vials from the freeze dryer during the drying process. These samples are subsequently analysed for moisture content. After the target water content is achieved, the drying phase is then concluded. The analysis of lyophiliser samples for moisture content can be a part of standard operating procedures at some laboratories; however, the process needs to be done carefully; otherwise, it can potentially compromise the sterility of the batch. Another issue with this procedure is that vials sampled at the front door are commonly observed to dry at a significantly faster rate than the vials in the centre of the tray, a phenomenon known as “atypical drying”. It is important to note that, as of today, there is no available technology or sampling of vials in large-scale freeze drying to determine the product’s secondary drying progress over time.
Validation of Lyophilisers in Life Sciences
In the life sciences sector, the validation of lyophilisers is essential for guaranteeing product quality and regulatory compliance. Validation is an approach that produces written evidence that assures a high level of confidence that a certain process will reliably deliver a product that conforms to its specified criteria and quality parameters. For lyophilisers, validation typically includes the following testing stages: Installation Qualification (IQ): This stage confirms that the lyophiliser and its components are installed accurately in accordance with manufacturer specifications and regulatory standards. It covers inspections of utilities, calibration certifications, and documentation. Operational Qualification (OQ): This step verifies that the lyophiliser functions as intended within its defined operating parameters. It involves assessing essential characteristics such shelf temperature consistency, vacuum efficiency, and condenser operation. Performance Qualification (PQ): PQ verifies that the lyophiliser reliably operates as anticipated under real production settings. This could involve executing test batches using sample products and showcasing consistency. Process Validation: This validation method establishes scientific evidence that the freeze-drying process can consistently deliver quality product. It typically involves running multiple batches and demonstrating that critical quality attributes are consistently met. Cleaning Validation: This ensures that the lyophiliser’s cleaning procedures are effective in removing product residues and preventing cross-contamination between batches. Computer System Validation: Modern lyophilisers with computerised control systems require validation of the software and control systems to ensure data integrity and reliable operation. The validation of lyophilisers involves ongoing evaluation and yearly requalification to guarantee continuous compliance and performance. This might include routine calibration of sensors, integrity evaluation of the vacuum system, and validation of essential process parameters.
Conclusion
Freeze drying is a complex process that has become essential in the biotech and pharmaceutical industries. By knowing the essentials of freeze drying, covering the initial freezing phase, and both primary and secondary drying, researchers and manufacturers may develop more effective and efficient methods for conserving sensitive biological materials. As the demand for protein therapeutics and other biopharmaceuticals, especially injectables, continues to grow, the importance of freeze-drying is likely to increase. Ongoing research and technological advancements aim to address current challenges, such as reducing cycle times and energy consumption, while maintaining or improving product quality and extending a shelf life. By mastering this sensitive balance and ensuring proper validation, the pharmaceutical industry can continue to produce high-quality, stable products that improve patient outcomes and advance medical care.
Bio Products Validation is a specialised provider of validation services to the UK and Ireland Life Science industries with expertise in the validation of freeze dryers. Should you need assistance with validation of your lyophilization processes or have questions, our experts are here to help. Please reach out to our friendly team using the contact form here.
Further Reading