Introduction
The pharmaceutical industry is experiencing a significant shift toward risk-based Commissioning and Qualification (C&Q), driven by the need for greater efficiency and streamlined validation processes. According to a recent ISPE survey, 68% of member companies plan to transition from traditional impact-assessment-based V-models to risk-based approaches. This transition, guided by ISPE Baseline Guide 5 2nd Edition, represents a fundamental change in how pharmaceutical companies approach facility and equipment qualification.
While risk-based C&Q principles have existed for years, adoption has accelerated recently as organisations recognise substantial benefits in cost reduction, timeline compression, and resource optimisation—all while maintaining regulatory compliance. This methodology aligns with current regulatory frameworks, including ICH Q9 Rev 1, ICH Q10, and FDA Process Validation guidance, offering a structured approach to modernising qualification practices. This article aims to discuss the history of risk-based qualification, it’s key components and the potential benefits and challenges when implemented.
The article draws from our insights gained from ISPE’s baseline guide 5 training programmes on Risk-Based Commissioning and Qualification methodologies and is intended for informational purposes only.
The Evolution of C&Q Strategies
Historically, the phases of engineering, construction, commissioning, and qualification were treated as separate, sequential steps. This approach often led to longer timelines and increased costs. In today’s pharmaceutical world, the pressure to get products to market on time ever increases, necessitating a new approach. Many guidance and regulatory documents have tried to introduce risk-based qualifications, such as ASTM2500, but haven’t given a full explanation of implementation. ISPE Baseline Guide 5 2nd Edition aims to tackle this.
Potential benefits of Risk-Based C&Q
There are many benefits to adopting this approach including:
- Resource Efficiency: Companies are reporting average budget savings of 2-3% per project.
- Reduced Documentation: Fewer validation documents are required.
- Faster Time to Market: The streamlined process shortens the overall project timeline.
- Regulatory Alignment: This approach aligns with current regulations such as ICH Q9 Rev 1, ICH Q10, ICH Q8, FDA PV guidance, and WHO guidance for qualification.
Key Components of Risk-Based C&Q
The methodology outlined views commissioning and qualification as an integrated process. This method focuses on:
- Emphasising facility and equipment design to prevent issues down the line using the Quality by Design approach.
- Eliminating the traditional IQ/OQ phases in favour of testing being done as part of the Factory Acceptance test (FAT) and the Site Acceptance Test (SAT). For this, it is important to truly understand the product and process and conduct an appropriate risk assessment to identify the Critical Quality Attributes (CQAs), Critical Process Parameters (CPPs) and Critical Aspects (CAs). Alongside the regulatory and site quality requirements, these can feed into the Critical Design Elements (CDEs) that will later be used in testing to verify the installation/ operation fit for the intended use.
- The level of effort in risk assessment should match the complexity and risk level of the process. For example, for complex and high-risk processes a FMEA may be needed. For simple off the shelf equipment, a less structured risk assessment can be conducted.
- Principles of Quality Risk Management (ICH Q9) should be operating throughout all stages of the project including validation.
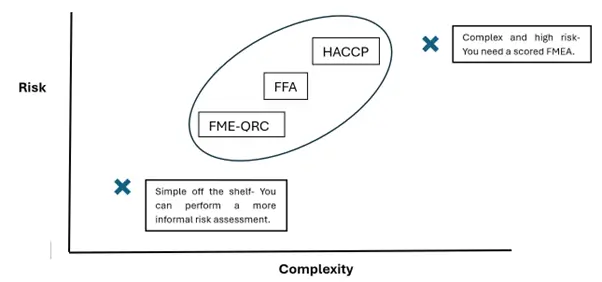
Graph 1- shows the relationship between risk level, process complexity and the suggested Risk Assessment Method.
What are the potential challenges when implementing risk-based qualifications?
While the benefits are clear, companies may face some challenges in adopting this new approach:
- Cultural Integration: This approach is a significant departure from previous approaches, so unless teams are educated on the benefits and regulatory alignment of the risk-based approach, there may be issues in the overall adoption of this strategy.
- Quality Concerns: Quality departments may be concerned that they may be cut out of projects because of the reduced qualification requirements. However, with this approach Quality Assurance is needed to be involved at an earlier stage, to promote quality by design in the planning and construction phase. There is also the potential added advantage of time savings for quality as there will be fundamentally fewer quality issues at the qualification stage. There is a need for education to prepare for the cultural shift needed.
- Digitalisation needs: To ensure optimal traceability, digitalisation of the C&Q process is vital. There are established solutions on the market such as KNEAT, Valgenesis and Sware, with many emerging providers also available. With this comes the need for cultural shifts to integrate these new technologies into current quality management systems.
Conclusion
The risk-based approach to C&Q represents a significant leap forward for the pharmaceutical industry. By focusing on critical quality attributes and regulatory requirements, companies can achieve greater efficiency without compromising on quality or compliance. As the industry continues to evolve, embracing this methodology will be crucial for staying competitive and delivering life-saving drugs to patients faster.
Further Reading
This article draws insights from ISPE’s baseline guide 5 training programme on Risk-Based Commissioning and Qualification methodologies. While specific training materials remain proprietary to ISPE, the key principles and implementation strategies discussed in this article align with ISPE’s current guidance and industry best practices. Organisations seeking detailed implementation guidance are encouraged to reference ISPE Baseline Guide 5 and related ISPE technical resources. Other relevant standards that may be of interest are ICH Q9 and ASTM E2500.
Bio Products Validation is a specialised provider of validation services to the UK and Ireland Life Science industries. Should you need assistance in determining if working with a validation service provider is right for your business please reach out to our friendly team using the contact form here.

